![[Return Home]]( http://www.forensic-applications.com/greybutton.jpg) | Forensic Applications Consulting Technologies, Inc. Click here for our Home Pages |
![[Return Home]]( http://www.forensic-applications.com/greybutton.jpg) | Forensic Applications Consulting Technologies, Inc. Click here for our Home Pages |
What do my samples really mean...?
1) Is sampling necessary?
2) What do the sample results mean?
3) Are my sample results high?
4) Are my sample results valid?
These are some of the common questions we receive from home owners and facility managers regarding the results of mold testing. The short answers are:
1) Almost never.
2) Probably nothing.
3) The results are probably uninterpretable.
4) Generally, "No."
With the elevated public awareness and unfounded fear of indoor molds has come a new type of consultant, the "Mold Inspector." Generally speaking, these consultants are poorly versed in microbiology, mycology, the occurrence of molds, their assessment, and their significance. Based on our experience, the credibility of the "certified mold inspector" is almost exclusively found in the collection of "samples" and the production of "laboratory reports." The samples collected by most "mold inspectors" are unscientific, uninterpretable, and largely meaningless, but which are often printed on impressive and official "Laboratory Reports."
Such lab reports are usually replete with fancy Latin names and apparently scientific notations. Some of the laboratory reports, such as reports involving the ServiceMaster R "InstaScope," the US EPA "Environmental Relative Moldiness Index (ERMI)" and the EMLab P&K MoldScoreTM, contain very elaborate and complicated and colorful tables, charts and other otherwise meaningless visual aids meant to impress those who don't realize the data are otherwise meaningless. (We will discuss the misrepresentations of some of these devices and protocols below).
It is the possession of the Laboratory Report that provides the credibility to the consultant; yet the true credibility of the Laboratory Report rests exclusively within the consultant's data quality objectives (DQOs) and the validity of the DQOs are incumbent on the expertise of the consultant. A "Laboratory Report" has no intrinsic value and the interpretation of the report can only occur in the context of the inspector's expertise, DQOs and hypothesis testing; indeed, the exact same air samples sent to different laboratories will produce wildly different analysis results,1a We recently (March 2022) encountered a Home Inspector with the home inspection franchise "Pillar to Post" performing sampling in an home. When we confronted him in the presence of the client, he admitted that he knew nothing about sampling or mold, but justified the scam by claiming that the experts at the analyzing laboratory (EMSL Analytical, Inc.) was interpreting the results for him. We explained to the Home Inspector and the client that is was impossible for a laboratory (EMSL Analytical or otherwise) to interpret the results.7 (It is important to note that there are no accepted "certifications" for mold inspectors or mold "testers" essentially anyone with a computer can sit down and print out a "certificate" on their home computer that "certifies" that person as a "certified mold inspector." In our experience, legitimate mold experts never use the term "certified mold inspector" "certified Microbial Consultant," "Certified Mold Remediation Expert" or any of the multitude of designations such as CRMI, CMI. These titles seems to be used exclusively by individuals who otherwise have no legitimate training in molds, and who typically are the ones who perform the majority of nonsensical sampling and "testing.")
Most "certified mold inspectors" believe they are collecting a sample to assess molds in an house. However, the "mold inspector" usually fails to meet the stated objectives in that the inspector fails to evaluate the building's fungal loading within any known degree of confidence, and usually relies on an unscientific and unfounded comparison of indoor to outdoor spore concentrations.
Some inspectors are even using "do-it-your-self" settling plate kits such as the IAQ Pro 5-Minute Home Mold Test, and the PRO-LAB Test Kits. This method is so grossly inadequate, it is not even considered sampling and is entirely incapable of producing valid results under all circumstances. In the settling plate method, Petri dishes are simply opened and exposed to the air for a period of time and then sent to a laboratory (usually the inspector's office). We have discussed these kits in greater detail below.
Many mold inspectors reference the US EPA document titled Mold Remediation in Schools and Commercial Buildings and identify the document as an applicable Industry Standard. Yet, in that document, the EPA recommends against performing the very kind of sampling conducted by most "mold inspectors." In the referenced document, the EPA states:
Is sampling for mold needed? In most cases, if visible mold growth is present, sampling is unnecessary.
The EPA warns:
Sampling for mold should be conducted by professionals with specific experience in designing mold sampling protocols, sampling methods, and interpretation of results.
In our experience, we have never encountered a mold inspector who has ever designed or used any kind of legitimate protocol with valid DQOs. In the EPA document the EPA states:
Sample analysis should follow analytical methods recommended by the American Industrial Hygiene Association (AIHA), the American Conference of Governmental Industrial Hygienists (ACGIH), or other professional guidelines.
However, the ACGIH, states that the primary emphasis on indoor mold assessments should rest with a thorough visual inspection of the property. 1
The AIHA 2 sponsored a publication titled "Recognition, Evaluation, and Control of Indoor Mold" 3 (generally referred to as "The Green Book.") The Green Book is an hodge-podge of conflicting presentations from various authors that presents both insightful, technically astute discussions alongside technically inaccurate and unsupportable myths and misconceptions. However, in the publication, the authors accurately state that a legitimate mold expert can adequately identify a mold or contaminated building material by merely looking at it.
Other professional guidelines, constituting industry standards, would include the 2000 publication titled Guidelines on Assessment and Remediation of Fungi in Indoor Environment (prepared by the New York City Department of Health, Bureau of Environmental & Occupational Disease Epidemiology), and considered by many industrial hygienists to represent a good practical approach. In that publication, the NYC guidelines states:
A visual inspection is the most important initial step in identifying a possible contamination problem.
Regarding air sampling, the NYC document specifically states:
Air sampling for fungi should not be part of a routine assessment. This is because decisions about appropriate remediation strategies can usually be made on the basis of a visual inspection.
The US Department of Health and Human Services, Centers for Disease Control (CDC) Mold Work Group report, 4 Chapter 2 " Assessing Exposure to Mold" states (in part):
Sampling for mold is not part of a routine building assessment. In most cases appropriate decisions concerning remediation and need for personal protection equipment (PPE) can be made solely on the basis of visual inspection. (sic)
The CDC document also recognizes the frivolity of the type of sampling normally conducted by "mold inspectors" when it stated:
Other than in a controlled, limited, research setting, sampling for biological agents in the environment cannot be meaningfully interpreted and would not significantly affect relevant decisions regarding remediation, reoccupancy, handling or disposal of waste and debris, worker protection or safety, or public health.
In the EPA document referenced above, the EPA states:
Inadequate sample plans may generate misleading, confusing, and useless results.For someone without experience, sampling results will be difficult to interpret. Experience in interpretation of results is essential.
Sampling should be done only after developing a sampling plan that includes a confirmable theory regarding suspected mold sources and routes of exposure. Figure out what you think is happening and how to prove or disprove it before you sample!
This is known as "hypothesis testing" and the sampling plan is a statement of "data quality objectives."
SAMPLING DATA QUALITY OBJECTIVES
As a professional standard, an investigator performing any kind of sampling should be capable of determining the errors and uncertainties associated with their sampling and, pursuant to standard industry practice, place the reported values in their proper perspective. This is the purpose of the "sampling plan" mentioned by the EPA and also mentioned by the Centers for Disease Control who stated: 5
If you do decide to pay for environmental sampling for molds, before the work starts, you should ask the consultants who will do the work to establish criteria for interpreting the test results. They should tell you in advance what they will do or what recommendations they will make based on the sampling results.
In our experience, we have never encountered a "mold inspector" that has ever established a priori criteria used for the collection or interpretation of their samples.
We frequently hear "mold inspectors" repeat the myth that sampling for molds is new, and as such, there are no standards to be followed. This is simply not true, since the concepts of sampling theory have been around for decades and apply to all kinds of sampling and human exposure assessments. In our corporate library, we still reference our original copy of William Wells' 1955 "Airborne Contagion and Air Hygiene" and the 1977 NIOSH "Occupational Exposure Sampling Strategy Manual", which clearly describe appropriate sample collection considerations.
Pursuant to decades-old, fundamental industrial hygiene (IH) practices and procedures, prior to the collection of any kind of sampling, the IH must establish a priori DQOs 6, otherwise the validity of the data is at best unreliable, and at worst, misleading. In our experience, all samples collected by Home Inspectors and "certified mold inspectors" on mold related projects were collected in the absence of DQOs, and therefore lack confidence, cannot be interpreted by anyone and are largely meaningless and misleading. This is not a disparaging remark about Home Inspectors. In our experience, Home Inspectors who perform visual inspections for molds are quite adequately trained to provide sound advice on the subject matter, provided they are not performing any kind of sampling. Also, in our experience, those Home Inspectors who perform mold sampling and mold testing are the least competent to perform mold consultation, and are often "certified" by a second rate laboratory which merely pushes sampling to increase its own revenues.
The establishment of DQOs is the quality assurance, quality control (QA/QC) part of a larger hypothesis testing or decision making process; the results of sampling and analysis will either support or not support the hypothesis but only in the context of the DQOs. This is part of the "sampling plan" mentioned by the EPA.
The DQOs ensure, through their prescription, that tenable and statistically valid results ensue. The DQOs ensure that a statistically sufficient number of samples will be collected from statistically representative locations in an acceptable manner by a validated method or method whose uncertainties are sufficiently characterized. The DQOs further specify that the samples are submitted to a laboratory capable of proficiently analyzing the samples to within a definable uncertainty, using valid methods.
The parameters of the DQOs themselves are based on statistical confidence needed to answer the a priori questions. The DQOs are what make laboratory results meaningful (and tenable). Without DQOs, one does not have data on a laboratory report; one has numbers and names on the laboratory report that cannot be interpreted by anyone (especially the laboratory), since the "numbers" have no intrinsic meaning outside of the context of the a priori decision criteria.
Furthermore, contrary to good science and standard assessment protocols, most "mold inspectors" leave the interpretation of their air sampling data to personnel at the analyzing laboratory. In our experience, Laboratory personnel never visit any of the subject properties from whence samples are collected, and have no knowledge whatever of site conditions. Permitting a laboratory to interpret one's analytical results has never been an acceptable industrial hygiene practice, and is almost exclusively used by consultants who lack the technical competency to interpret their own samples. According to the US Centers for Disease Control: 7
The results of samples taken in your unique situation cannot be interpreted without physical inspection of the contaminated area or without considering the building"s characteristics and the factors that led to the present condition.
The DQO process is so entrenched in good environmental sampling that it is the underpinning of all such sampling and is discussed in great detail in many broad-spectrum environmental sampling protocols and Industrial Hygiene sampling protocols. For example, one of the "bibles" of general environmental sampling is the US EPA SW846 8 geared toward environmental sampling. The sampling precepts and the QA/QC foundations are recognized as being applicable to all kinds of sampling. The SW 846 describes DQOs thusly:
Data quality objectives (DQOs) for the data collection activity describe the overall level of uncertainty that a decision-maker is willing to accept in results derived from environmental data. This uncertainty is used to specify the quality of the measurement data required, usually in terms of objectives for precision, bias, representativeness, comparability and completeness. The DQOs should be defined prior to the initiation of the field and laboratory work. The field and laboratory organizations performing the work should be aware of the DQOs so that their personnel may make informed decisions during the course of the project to attain those DQOs.
Interpreting Data: PARCC Parameters
From an industrial hygiene perspective, the interpretation and reporting criteria for airborne constituents, including moulds, are commonly referred to as "PARCC" parameters: (precision, accuracy, representativeness, comparability, completeness). At the end of the sampling and testing, the IH should be able to answer each of the PARCC parameters.
Precision:
How reproducible are measurements; has the variance been characterized?
Axiom 1: All samples exhibit uncertainty.
Axiom 2: All analysis results exhibit uncertainty.
These statements are true regardless of the parameter being sampled or analyzed. The precision of the data collected by "mold inspectors" is entirely unknown because "mold inspectors" and Home Inspectors generally fail to develop and/or follow a sampling plan that would determine the precision. It is an established and industry accepted fact that particle migration is mainly influenced by particle properties, ventilation conditions and airflow patterns. 9 Particle concentrations in general, 10 and spore concentrations in particular 11 within a structure exhibit extremely large spatial variations 12 which tend to be compartmentalized within a given space.
This is to say that if an inspector were to lay out ten identical samplers within a room, and collect ten identical samples at exactly the same time, they would end up with ten completely different sample results; the spore counts would be wildly different and even the types of organisms identified would be wildly different. Yet, each sample came from the exact same room at the exact same time!
Furthermore, the air samples collected by "mold inspectors" are exclusively short-term samples (three to ten minutes in duration). It is a well established and standard sampling precept that short term samples exhibit extremely large temporal variations. 13
This is to say that if an inspector were to collect ten identical samples within the same room, but at different times of the day (or year), they would end up with ten completely different sample results; the spore counts would be wildly different and even the types of organisms identified would be wildly different. Yet, each sample came from the exact same room!
We can speak of the variability in terms of "deviation" which indicates the amount of "spread" of results about an "average" concentration (actually a "mean" concentration). Generally, the geometric standard deviation (GSD) of interday and intraday airborne concentrations lie between 1.2 and 2.5 geometric standard deviations. 14 These large variations are similar to those seen by other authors, specific to airborne mold concentrations 15,16,17some of whom have reported even higher fungal variations in indoor air. 18
We have prepared a detailed discussion of this issue and the problems overlooked by "mold inspectors" which can be found by clicking here.
Classic industrial hygiene sampling strategy indicates that reasonable confidence in estimating an average ambient airborne concentration is achieved when at least 70% of the exposure time is measured, 19 and states that random "grab samples" are the least desirable technique for estimating the average exposure. 20 It is in fact "grab samples" that are exclusively collected and reported by "mold inspectors" during their "mold surveys." In our experience, "mold inspectors" exclusively use single random grab sample methods from selected areas (including outdoors). The total sampling time for each grab sample is usually much less than 1% of the anticipated exposure time for the exposed population (the home owner or building occupant). This error is known as the "sampling design error," and if uncharacterized produces huge uncertainties in the reported results.
Although considered a "counsel of perfection," a concept called "Shannon's Sampling Theorem" 21 estimates that the number of measurements needed to achieve "perfect information" about airborne concentrations is roughly 250,000 measurements per cubic meter per hour. More practical foundational and accepted classic industrial hygiene references22,23 have estimated that for each daily study period (usually expressed as any eight hour period for a work place or 12 hours for a residential setting) between eight and eleven random grab samples are needed from each study area (each room, crawlspace, outdoors, etc.), to obtain adequate confidence in determining the variance associated with the study area for that day. Without knowing the variance, one cannot know the average concentration, and certainly one cannot know the average concentration based on a couple of samples.
Some authors24 state that as many samples as necessary to determine the distribution should be taken. As such, one sample (or indeed two or three) collected from each study area, such as those collected and reported by Home Inspectors or "certified mold inspectors" cannot provide adequate confidence in estimating the spore concentration in a subject property.
It is a well established fact that spore counts of airborne fungal entities exhibit a lognormal distribution throughout the day. 25 This means that the variation between one or two samples can be huge and skewed in one direction. 26 As an example, the following spore trap data are real data and are very typical and from a Colorado home.
Air Monitoring Data
| Time of Sample | Spore Count |
| 08:00 | 213 |
| 09:30 | 1,195 |
| 11:00 | 393 |
| 12:30 | 567 |
| 14:00 | 900 |
| 15:30 | 3,257 |
In viewing this table, one may ask: "Which sample represents the spore concentration for the home?" Answer: They all do; they are all correct, and none of them are contradictory. So if mold testing personnel collected the sample at 08:00, they would very likely have a different conclusion about this home than if they collected the sample at 15:30. And yet, these are the same variations expected to be present at all study locations. Samples collected pursuant to proper DQOs will define the variance and determine the validity of any one, or a set, of sample data. 28
It is for this reason, the airborne concentrations for a study area cannot be adequately characterized by collecting just a few samples. Due to the temporal and spatial lognormal distribution almost always seen in indoor and outdoor spore concentrations, and the short sample time29 associated with sample collection techniques, the single short-term samples collected in each study area have a low probability of representing the actual airborne fungal loading in that area, in the home in general or outdoors.
Remarkably, contrary to simple mathematics, most "mold inspectors" and other poorly trained consultants attempt to derive the arithmetic mean from their samples. An arithmetic mean would be appropriate if the data exhibited Gaussian distribution. However, most "mold inspectors" never determine the distribution, and there is no reason to believe that the distribution would be Gaussian or anything other than lognormal. 30, 31 Even taking the mean of the log transformed data would be a better representation of an "average," 32 but would still only approximate the median value and not the true "average."
As it is, the intrinsic variability of the air samples is already very high, which by itself results in a significant obstacle to interpretation. But that does not even take into account the extrinsic variability seen amongst the different laboratories performing the analysis.
If a "mold consultant" were to collect ten air samples from a property, and sent those samples to an AIHA Accredited Laboratory for analysis, but didn't like the analytical results, that consultant could have the samples sent to another AIHA accredited laboratory and receive a different laboratory report with completely different results. If the consultant still like the results, he could send the samples to another and yet another laboratory until he finally received results he liked and those results would be in the form of a legitimate laboratory report from an accredited laboratory.
This is because the interlaboratory variability is also huge, and the exact same samples sent to different accredited laboratories can result in analytical results that span greater than an order of magnitude difference. 33a
Summary of Precision
The air samples collected by "mold inspectors" are usually entirely unusable since there is no confidence in the data based on precision.
Accuracy: Accuracy asks "How close is the reported value to the true value? "
The lack of precision associated with reported data would shift the emphasis for ensuring confidence toward accuracy. That is, in the absence of precision, one may rely upon highly accurate data to aid in the interpretation of the data. Each sampling method has only a limited inherent ability to enumerate specific types of spores. The types of samplers used by most inspectors are spore traps (such as the Air-O-CellTMcassette), and it's collection efficiency is very well established. 33
Each sampler has a specific and known physical limitation known as the "cut-size." The "cut-size" is the aerodynamic diameter, in micrometers of a theoretical spherical particle of unit density that has a 50% chance of being captured and is designated "d50."
Generally, at the recommended flow rate of 15 liter of air per minute, at normal temperature and pressure, the d50 for the Air-O-CellTM cassette is reported as 2.5 µm. 34 This means that a mold spore whose diameter is approximately 2.5 µm has only a 50% chance of being captured. Most of the fungal conidia found in indoor environments are at or near this aerodynamic diameter; therefore, immediately, the accuracy of the results must have error statements wherein the confidence of the result lies within one half to twice the reported values. This is not a flaw with the sampling devices, but rather places additional emphasis on the expertise of the consultant to understand what the limitation means, and design DQOs that will take the cut-off values into consideration.
Having said this, often then samples were not collected under standard conditions, since in many situations, the samples are collected at altitude (such as in Denver) or at very warm or very cold temperatures. Each deviation from the ideal temperature at sea level shifts the d50 one way or another. Although some early authors suggested that real collection efficiency curves may be approximated with a sloping straight line (which would aid in increasing the interpretive value of the reported data), more recent information indicates the collection efficiency is much more complex and as sampling altitude increases, and/or the sampling temperature increases, the cut-size also increases; as the airflow rate through the sampler increases, the cut-size decreases35 and even more curious, the actual effective cut-size for the common slit impactor spore trap can change as the mixture of spore sizes changes. 36
The net result is that the untrained "mold inspector" doesn't realize that a spore trap result of say, 1,000 spores per cubic meter of air could come from an atmosphere containing fewer spores than a sample collected by an identical spore trap of the same area with a result of say 500 spores per cubic meter of air. 37 This is because the "total spores" reported on the laboratory report is not the actual total loading per unit of air, but is rather just a representation of the proportion of those spores which may have been trapped which changes for each spore type.
To illustrate this point consider the following scenario (See the data table below)- The air in an home contains the spores from 12 different kinds of fungi. Each genus and species produces spores in a narrowly defined size range, so the spores from each species is a different size.
Spore Size Effects on Air Monitoring Data
| Species | Spore Size (µm) | Collection Efficiency | Scenario #1 Actual Spore Concentration | Scenario #1 Spore Concentration Reported by Lab | Scenario #2 Actual Spore Concentration | Scenario #2 Spore Concentration Reported by Lab | |
| Spore A | 1 | 0.1 | 100 | 10 | 520 | 52 | |
| Spore B | 30 | 0.9 | 100 | 90 | 29 | 26 | |
| Spore C | 1 | 0.1 | 100 | 10 | 389 | 38.9 | |
| Spore D | 2 | 0.3 | 100 | 30 | 289 | 86.7 | |
| Spore E | 1.5 | 0.2 | 100 | 20 | 378 | 75.6 | |
| Spore F | 5 | 0.7 | 100 | 70 | 37 | 25.9 | |
| Spore G | 2 | 0.3 | 100 | 30 | 198 | 59.4 | |
| Spore H | 10 | 0.7 | 100 | 70 | 41 | 28.7 | |
| Spore I | 2.5 | 0.5 | 100 | 50 | 48 | 24 | |
| Spore J | 2.5 | 0.5 | 100 | 50 | 60 | 30 | |
| Spore K | 15 | 0.9 | 100 | 90 | 22 | 19.8 | |
| Spore L | 1.5 | 0.2 | 100 | 20 | 365 | 73 | |
| Total spore concentrations | 1,200 | 540 | 2,376 | 540 |
Now, in the first scenario, the spores from each genus are present at exactly 100 spores per cubic meter of air. Therefore, there are 1,200 spores present per cubic meter of air. However, since the sizes of the spores are different, the collection efficiency of each species is different, and so the proportion of spores trapped and retained is different. In this case, the laboratory analyzes the sample and reports finding 540 spores per cubic meter of air. The laboratory report is correct! Now it is up to the consultant to understand why 540 spores per cubic meter of air could have come from an atmosphere containing 1,200 per cubic meter of air.
But spore concentrations don't neatly appear in equal concentrations, rather, they are present in unpredictable proportions (called a profile). So in Scenario Number 2, the air in the house contains a mixture of spores present at different concentrations. The total concentration of the spores in the air is 2,376 per cubic meter of air. But because of the collection efficiency of the spore types present at the specified profile, the laboratory still reports the concentration as 540 spores per cubic meter of air. The laboratory report is right! Now it is up to the consultant to understand why a laboratory report stating 540 spores per cubic meter of air could be from an atmosphere containing either 540 spores or 1,200 or 2,376 or ... ? It is likely that the first time a "certified mold inspector" has any idea that this is happening to their samples is when they read this page.
This problem with accuracy is inherent to the samples themselves - but also not recognized by "mold consultants" is that the laboratories themselves have difficulty in analyzing the samples accurately. That is different laboratories analyzing the exact same samples report different kinds of mold being present.
One study from 2011,37a
revealed that only 75% of the accredited laboratories could consistently identify Cladosporium, the most common mold in the environment. Furthermore, Aspergillus/Penicillium-like spores, the most common mold category related to water intrusion, were identified by only 50% of the accredited laboratories.The authors concluded:
This research reveals that precision of spore trap analyses, even among laboratories involved with analytical proficiency testing, lack precision and should be interpreted with caution.
Summary of Accuracy
The up-shot is that not only is the precision of the data generated and reported by "mold inspectors" extremely poor, the accuracy of the data is also extremely poor due to the inherent limitations of the samplers used in the absence of DQOs. We have never encountered a mold inspector who actually understands that the accuracy of their data is very limited. In our experience, "certified" mold inspectors view the laboratory report as some kind of magical document which represents absolute truth. In fact, the laboratory report is a meaningless document, if the consultant cannot place the values within the context of his Data Quality Objectives.
Relevancy: Relevancy asks: "Do the data speak to the a priori question being asked?"
Contrary to good sampling protocols, such as described by Dr. Harriet Burge, 38 in our experience, we have never observed where a Home Inspector or "certified" mold inspector has actually asked an a priori question, developed an hypothesis to test, or provided any point of relevancy to the collection of their samples.
Indeed, based on the conclusions provided by most "mold testers," none of the samples or sample results provide any information that was not already known prior to sampling, but frequently provide misleading conclusions that are not substantiated by valid samples.
In their reports, Home Inspectors and mold testers never actually explain how their samples are used or could be used to support their conclusions.
Comparability (Points of reference):
Comparability is the reference point against which one may answer the question "Are the results high?" Low? Normal? Abnormal? According to whom? By which metric/standard? Is the metric or standard accepted, and if so, by whom?
Decisions about air contaminants can often be based by comparing the qualified results against a regulatory requirement, nationally accepted guideline, or confidently characterized guidelines. As stated in the EPA recommendations, those criteria should be explicitly identified prior to the collection of the samples. We have never seen where a mold inspector exercised any valid comparisons, but, as already mentioned, most inspectors rely on popular myth and misconceptions (such as comparing indoor to outdoor concentrations) or other sloppy thinking to interpret the data.
In their reports, most "mold inspectors" use a method of comparison that is popular amongst poorly trained consultants who presume their single indoor datum is valid, and is then compared to a single outdoor datum, which is similarly presumed (erroneously) to be valid.
However, this comparison is generally considered argumentum ad populum in the light of state-of-knowledge. Essentially the consultant makes the case that "... since everyone seems to be doing it, it must be correct." In fact, in making the comparison, "mold inspectors" never provide any support for the comparison, but rather shift the responsibility of interpretation to the analyzing laboratory.
The previously referenced EPA document explicitly states:
The results of samples taken in your unique situation cannot be interpreted without physical inspection of the contaminated area or without considering the building's characteristics and the factors that led to the present condition.
We have never seen a situation where laboratory personnel visited a study site and performed a physical inspection. Therefore, laboratories have absolutely no basis to perform the interpretation and therefore, usually only lower quality labs engage in this kind of behavior.
It has long been known that there is no correlation between indoor and outdoor spore concentrations in the circumstances under discussion, and investigators who practice such indoor/outdoor comparisons are exclusively poorly trained practitioners who lack a legitimate understanding of indoor aerobiology.
It is possible that the myth regarding indoor v. outdoor comparisons started with notable, well respected researchers who alluded to indoor/outdoor generalities39 and those generalities were then taken out of context and referenced inappropriately in subsequent texts.
For example, in the 1998 edition of NIOSH's Manual of Analytical Methods, QA/QC Chapter J, NIOSH40 partially quoted a reference and stated:
In general, indoor microflora concentrations of a healthy work environment are lower than outdoor concentrations at the same location.(Macher & Burge 1995) If one or more genera are found indoors, in concentrations greater than outdoor concentrations, then the source of amplification must be found and remedied.
In the above statement NIOSH references the source as: Macher JM, Chatigny MA, Burge HA [1995]Sampling airborne microorganisms and aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617.
However, if one goes to the original source (Macher & Burge, 1995), we see that the referenced material does not support the statement as presented. The authors Macher & Burge made the first observation (the general comment about indoor v. outdoor concentrations), but did not make the et sequitur conclusion ... rather that was an unsupported misinterpretation by NIOSH.
Placing the comments of the original cited authors back into context challenges the fundamental legitimacy of performing indoor/outdoor comparisons and is contrary to what the author wrote elsewhere on indoor/outdoor concentration issues wherein the same original author (Burge) also in 1995, observed: 41
Indoor/outdoor relationships: Unless there is an indoor source for specific bioaerosols, concentrations indoors will generally be lower than outdoors. This effect is related to the reasons for occupying enclosures, which are designed to protect us from adverse weather and intrusion by vermin or other unwelcome (sometimes human) visitors. The outdoor aerosol penetrates interiors at rates that are dependent primarily on the nature of ventilation provided to the interior. Indoor/outdoor ratios of specific particle types (of outdoor origin) are highest (tending toward unity) for buildings with "natural" ventilation where windows and doors are opened to allow entry of outdoor air along with the entrained aerosol As the interior space becomes more tightly sealed, the ratio becomes lower and lower.
Therefore, the indoor/outdoor ratio of airborne moulds is primarily a function of building systems, and the indoor to outdoor ratio will rise and fall with the normal ventilation infiltration rate and other factors not related to indoor mold growth. Unless one has evaluated the infiltration/exfiltration characteristics for the structure, one cannot know how close to unity one may expect the structure to be (even if one has data that exhibits statistical confidence).
Over the course of time, untrained mold inspectors have repeated the NIOSH quote which has grown out of context and is now misconstrued to a point of perverse "normality" exclusively through tautology, but the oft repeated sentence still remains without foundation.
As mentioned, the spatial and temporal variations in spore concentrations already described are equally large outside as inside (but for slightly different reasons). Furthermore, the concentrations of outdoor spores vary enormously with species, location, altitude, season, climate and time of day, and indeed, many organisms exhibit relatively predictable increases and decreases with time of day. 42
Therefore, similar to indoor samples, unless one has collected a sufficient number of samples to properly characterize the outdoor population distribution, one lacks the necessary precision to compare that sample with a contemporaneous indoor sample.
That is - while the indoor spore concentrations (and specific genera and species) are fluctuating wildly, the outdoor spore concentrations (and genera and species constituents) are also fluctuating wildly, but at different times, in different locations and for different reasons.
Furthermore, when legitimate sampling protocols, such as those found in official NIOSH reference documents, make allusions to the comparisons of indoor to outdoor concentrations, 43 they axiomatically are indicating that one has actually measured, with confidence, the actual concentration used in the comparison, and not simply taken one or two or three unreliable grab samples. Grab samples merely represent a "snap-shot" and not the overall concentration. Therefore, where NIOSH recommends comparing indoor to outdoor samples, they also state: 44
Select at least three sites, one each to represent complaint area, a noncomplaint area and outdoors.
In turn at each site, sample simultaneously for fungi, mesophilic bacteria, and thermophilic actinomycetes.
Before moving to the next site, repeat twice to obtain triplicate, consecutive samples.
Collect another complete set of samples and blanks on the next day.
Therefore, at the end of the sampling period, the consultant would have collected six samples for fungi, mesophilic Bacteria, and thermophilic actinomycetes from each study area, six samples of the same from an indoor control area and six samples from the outside. This is a far cry from the protocol usually seen with "mold inspectors" collecting one or two meaningless samples from an indoor area and comparing that with one or two meaningless samples (inappropriately "averaged") from the outdoors.
In the earlier referenced document,45 the EPA states:
Keep in mind that air sampling for mold provides information only for the moment in time in which the sampling occurred, much like a snapshot. Air sampling will reveal, when properly done, what was in the air at the moment when the sample was taken. For someone without experience, sampling results will be difficult to interpret. Experience in interpretation of results is essential.
We know that if one collects a three minute sample from a specific location, three minutes later, the airborne spore count will be wildly different in the same location. In the table below, we have presented very typical instantaneous indoor/outdoor collocates taken from a normal Colorado home.
Air Monitoring Data
| Time | Indoor Spore Count | Outdoor Spore Count |
| 10:00 | 971 | 6 |
| 13:15 | 16 | 112 |
| 15:23 | 33 | 102 |
| 18:06 | 426 | 133 |
As can be seen from the above data, the indoor counts are showing the expected large variation (lognormal distribution), and simultaneously, the outdoor samples are similarly showing their expected large variation (lognormal distribution).
Comparing one-on-one samples is a meaningless pursuit; and, as demonstrated in the typical example above, if one used that decision criteria, the home in the above example would have "elevated" spore counts (and thus a mold problem) at 10:00 a.m. and 6:00 p.m, but would be considered normal at 1:15 p.m. and half past three in the afternoon.
In a similar example, the table below presents another actual data set from a normal residential setting in Colorado during the month of July.
Air Monitoring Data
| Time | Data Set A | Data Set B | Data Set C |
| 08:45 | 419 | 1,306 | 2,629 |
| 10:20 | 290 | 4,4,84 | 3,000 |
| 12:12 | 452 | 2,306 | 3,000 |
| 14:15 | 484 | 14,065 | 3,468 |
| 16:16 | 742 | 7,339 | 3,048 |
| 16:45 | 210 | 5,290 | 3,242 |
| MVUE | 434 | 5,858 | 3,064 |
In this case, a "Toxic Mould Expert" (who is still practicing mumbo-jumbo mold testing) had frightened an elderly couple with slit-impactor sampling methods. The "consultant" managed to frighten the couple into leaving their home. FACTs performed scientifically valid sampling in the "contaminated" home, in the outdoors and in another home in the area (not experiencing any mold related problems) as a control. The windows and doors in the study home were closed and the occupants maintained closed-building conditions. The occupants of the control home kept the all the windows in the building open during summer months. The MVUE47
listed in the table is the actual "average" based on the lognormal distribution. The "MVUE" is the statistic considered to best represent a point estimate of the true "mean" for lognormally distributed values. The MVUE is preferred over the geometric mean, especially when sample populations are small.Do the data in the above table intrinsically identify which home had a mold problem? Or do the data identify which home was the healthy control home or which data set represents the outdoor samples? No. In the above table, Data Set A was collected from the "contaminated" home; Data Set C was collected from the healthy control area; and Data Set B were samples collected from the outdoors.
Regarding indoor versus outdoor comparisons, too many broad statements are made without due consideration for building conditions and regional and microclimate changes which can greatly alter the variations in concentrations and can greatly alter the relationship between the indoor environment and the outdoor environment.
For example, in Colorado our seasonal changes are so large, that on some days, we may open our doors and windows permitting not just direct communications between indoor and outdoor spore concentrations, but actual equilibrium of the outdoors spore concentrations with the indoor air spore concentrations. 48
Extensive data collected by FACTs indicate that for samples collected under "closed building conditions," regardless of the region, there is poor correlation49 between indoor and outdoor fungal profiles (genera, species and total counts) whether the building is a "problem" (symptomatic) building or a "healthy" (non-symptomatic) building. Studies by other researchers5 have made similar conclusions regarding outdoor versus indoor influences, particularly with regard to particulates. 51
In the graphic below, we have presented data for indoor and outdoor fungal concentrations for both symptomatic (problem) buildings and non-symptomatic (healthy) buildings collected in Colorado. In the following graphic, each vertical bar indicates a single house, and the vertical bar is the MVUE derived from at least six samples collected from the structure. These findings are similar to that reported elsewhere in the literature. 52
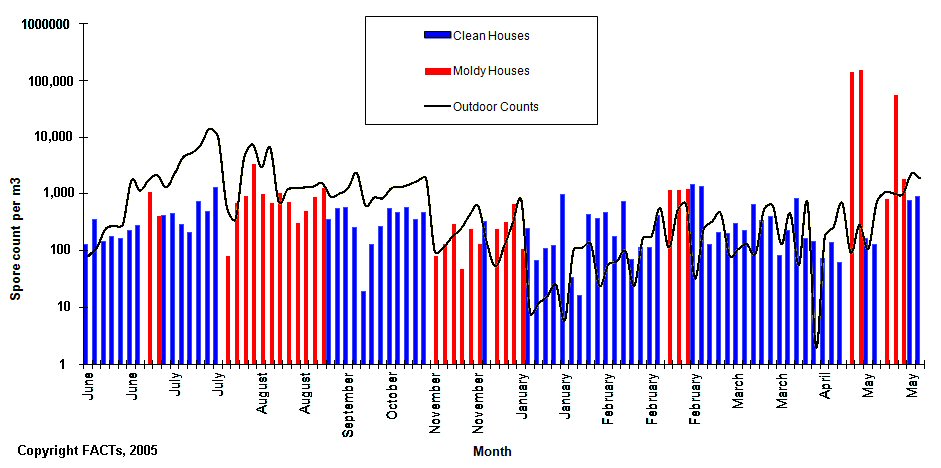
As can be seen from the above graph, there are clearly times when "problem" homes have spore concentrations less than outdoor concentrations and when healthy homes have spore concentrations higher than outdoors. Some studies purporting to demonstrate correlations between indoor and outdoor air, 53
however, exhibit fatal flaws upon closer scrutiny, and those studies do not survive scientific rigor. 54Furthermore, in temperate zones it is a well accepted fact that outdoor spore concentrations change dramatically with seasons, and exhibit a bi-modal distribution whose peaks are in the early spring and early autumn. And although our definition of season (summer, autumn, etc.) is based upon the equinoxes, moulds, being living organisms, are more interested in mean diurnal temperatures, precipitation levels, sun light cycles, relative humidity and so forth, for their living and growth conditions.
In the data set graphic below, for Colorado, winter outdoor MVUE fungal counts are about 209 spores/m3, with individual counts exceeding 900 spores/m3 approximately 10% of the time. Spring counts are approximately 900 spores/m3 with individual counts exceeding 900 spores/m3 approximately 40% of the time. The summer counts average about 3,500 spores/m3 with individual counts exceeding 900 spores /m3 approximately 72% of the time. And yet, non-symptomatic closed-mode indoor counts remain roughly the same throughout the year (horizontal central line).

Therefore, in Colorado, the representative indoor spore count in normal, healthy houses will exceed the outdoor counts almost four months of the year. Laboratories and consultants, who claim that a problem exists if the indoor counts exceed outdoor counts ignore the fact that the spore counts in healthy houses stay relatively stable, but the outdoor seasonal counts fluctuate around the indoor concentrations. Therefore, according to the criteria found in some "standards" (such as the IESO "standard," which is not recognized by legitimate microbiological experts as a "standard"), healthy houses contain excessive levels of moulds in the winter but are normal in the summer, even though the spore counts in the houses may not have changed.
Most "mold consultants" make a special point of noting that the genus Stachybotrys may be identified in the indoor sample. Stachybotrys is the "bogeyman" used by the toxic mold charlatan to frighten people, and its presence is notable only to those who don't realize that it is ubiquitous in ALL houses.
Summary of Comparability
The comparisons made by mold testers are usually entirely untenable and the conclusions drawn from those comparisons, are usually without validity.
FACTs has performed valid air sampling and developed an extensive database of spore counts that permits direct comparison of other valid samples. Based on our in-house database of total fungal counts (spores and fragments), as determined by the spore trap Air-O-CellTM sample method, the fungal counts for indoor samples in temperate zones, for closed building conditions 55, in buildings not experiencing fungal problems usually have an MVUE of less than about 500 spores per cubic meter (spores/m3). However, even in those properties, the indoor concentrations exceed 900 spores/m3 12% of the time. This means that even in perfectly clean houses, during closed-mode conditions, one out of every 10 samples will exceed 1,000 spores per cubic meter of air and a finite probability that any one sample will exceed any given elevated value.
By contrast, the MVUE total fungal counts in buildings with fungal problems, is greater than 40,000 spores/m3 and fungal concentrations exceed 900 spores/m3 66% of the time. 56
Essentially, this means that in even the "cleanest" house with no mold problem, there is a finite and high probability that a single sample (or two or three) will exceed even, say, 3,000 spores per cubic meter of air; and that in even the moldiest of houses, a single sample may be as low as 3,000 spores per cubic meter of air.
At this point, it is probably important to address the following myth:
Myth Number 1: Airborne spore concentrations correlate to the amount of mold present in a building.
Truth Number 1: There is NO correlation between air concentrations and the amount of mold, hidden or otherwise in a building.
Some of our lowest air sample sets have come from properties with thousands of square feet of visible, actively growing molds. Some of our highest data sets have come from properties with NO mold problems, (hidden or otherwise).
This makes sense, since it is a myth that the number of spores present in an area is somehow related to the size of the vegetative mass ... but that simply isn't true. As a mold is actively growing, and using its resources (food and water), it is in a mad rush to colonize and fight off competition ... it doesn't have time to be engaged in reproductive activities! Result: Lots of vegetative mold growth, not many mold spores in the air.
Air sampling, even properly conducted air sampling is entirely incapable of quantifying the amount of mold in a structure and is entirely incapable, except under extremely unusual circumstances, consisting of some very stringent DQOs, of confirming the presence of "hidden" mold. Generally, these kinds of assessments are very expensive, and virtually never needed.
The American Academy of Allergy and Immunology (AAAI) classifies general allergic responses as follows: 57
AAAI Classification
| Total Spore Count | Classification | Allergy sufferers who are allergic to these pollens or moulds may experience symptoms of hay fever |
| 0 | Absent | No Symptoms |
| 1- 6,499 | Low | Only individuals extremely sensitive to these pollens and molds will experience symptoms |
| 6500 - 12999 | Moderate | Many individuals sensitive to these pollens and molds will experience symptoms. |
| 13000 - 49999 | High | Most individuals with any sensitivity to these pollens and molds will experience symptoms. |
| 50000 | Very High | Almost all individuals with any sensitivity at all to these pollens and molds will experience symptoms. Extremely sensitive people could have severe symptoms. |
Therefore, in comparing indoor to outdoor, and maintaining the position that the indoor environment does not have a mold problem if indoor spore concentrations are less than outdoor spore concentrations then, according to these same inspectors, indoor concentrations should not be of concern until about 13,000 spores per cubic meter. Other researchers have reported "allergic thresholds" for specific molds such as 3,000 CFU/m3 for the ubiquitous genus Cladosporium.58
We do not see how "mold inspectors" maintain these conflicting and incongruous positions in the face of overwhelming published data, accepted knowledge, logic and common sense to the contrary.Completeness: Have all the DQOs been met; i.e. are the data reliable and do they exhibit confidence?
Since no DQOs are ever established by "mold inspectors", no DQOs can be met.
Hidden Mold
There is a misconception that mold may be hidden in wall cavities and that the hidden mold presents an exposure problem. However, mold hidden in wall cavities does not present a significant exposure threat. FACTs has performed several hundreds of building assessments for molds and Bacteria. We have never encountered a single project where sampling (air sampling or bulk sampling) was successful in discovering a hidden mold problem that was overlooked by normal, thorough visual inspection.
Furthermore, we have never encountered a situation where mold hidden in wall cavities that ever resulted in the degradation of the indoor air in a structure. When the front door of a residence is opened on a nice spring day in a temperate zone, approximately two cubic meters of air are displaced and mixed into the structure in an instant. At a moderate spore concentration of just 5,000 spores per cubic meter, the occupant has just introduced 10,000 mold spores into the home in a matter of seconds.
By contrast, based on our tests using ventilation fume at various penetrations, the air movement through a wall cavity, capable of carrying mold spores or other fungal entities is so small as to be entirely insignificant by comparison.
Since the route of migration is insignificant, the source, however large or small, becomes unimportant since there is no reasonable way for the source to get to the recipient. In any event, in general, it is recognized by the cognizant scientific community that colonization of mold inside wall cavities does not present an exposure issue : 59
... it is reasonable to infer that small amounts of mold enclosed in walls, floors, or ceilings will not have a large impact on the indoor air quality.
The Wisconsin Department of Health and Family Services investigated the relationship between mold on surfaces of oriented strand board siding and mold levels inside the home; the results of the study indicated mold levels in the affected homes were not significantly higher than those measured in "non-exposed" homes. 6
As such, to our knowledge, there is no compelling reason to address mold inside wall cavities based on mere presence. This is consistent with the opinion of one of the world's leading experts on mold, Dr. Harriett Burge who stated: 61
However, removal based on the mere fact of its presence, or based on nonspecific symptoms that are not related to mold exposure, is often not appropriate.
We have seen several studies wherein authors have claimed that air monitoring or other sampling techniques have discovered hidden molds. However, upon review of the studies, the air monitoring in those cases were usually poorly and improperly conducted and merely augmented the visual inspections, and in no cases discovered hidden mold that would not have been otherwise found by a normal visual inspection performed by an experienced investigator. One of the authors62 of the The Green Book similarly makes this observation:
Some investigators have used air samples collected inside wall and ceiling cavities to document the extent of hidden mold, but this technique is controversial and interpretation is uncertain.
And elsewhere, the author explains that:
Finding hidden mold is difficult and expensive. If there are no smells (sic) no complaints, and no indication of significant moisture damage, we can reasonably be sure that there is no problem and no reason for further investigation.
Studies and investigations performed by this author (Connell), consistent with other researchers, have not observed a correlation between mold hidden in walls and a degradation of indoor air quality or a correlation between mold hidden in walls and an increase in spore counts in occupied spaces. Even properly conducted airborne mold sampling is generally incapable of determining when hidden mold may be present.
FINAL CLEARANCE SAMPLING
FACTs has been involved in mold remediation and correction activities for hundreds of residences and commercial buildings. We have never seen a single situation where a mold inspector performed proper sampling techniques for final clearance activities. Furthermore, we have never seen a single situation where sampling, including properly conducted sampling, has ever demonstrated the efficacy of a mold remediation activity that was not readily known by a visible inspection. We have discussed the myths of mold remediation, decontamination and clearance sampling here.
A recent (November 2012) NIOSH document 62a addresses the "clearance sampling" issue thusly:
Building consultants often recommend and perform "clearance" air sampling after remediation work has been completed in an attempt to demonstrate that the building is safe for occupants. However, NIOSH does not recommend this practice, as there is no scientific basis for the use of air sampling for this purpose.
In short, there is no such thing as valid "clearance testing" for molds, as commonly conducted by "mold inspectors" and mold remediators. No such tests, as commonly conducted, are scientifically valid, and none stand up to scientific scrutiny. The Green Book addresses "final clearance sampling" thusly:
This perspective has been the central opinion of the knowledgeable body of science for approximately 20 years. We typically only see "Certified Mold Inspectors" (CMIs) and other poorly trained individuals performing these misleading clearance sampling schemes. 18.5.2
Current mold remediation guidelines support the concept that project success depends on verification primarily through inspection that visible mold growth and associated debris and dust were appropriately removed63,
The AIHA publication continues with:
The primary objective of mold remediation, based on guidelines published between 199366 and 200467 68 is to remove visible mold growth and return material surfaces to a satisfactory condition.
The section concludes with:
A difficulty associated with using air sampling as the primary means of achieving final clearance is the absence of numerical guidelines for airborne fungi and for bioaerosols in general. 69, 70, 71 IOM72 concluded that, although there is an association between respiratory health effects and dampness, the exact causal agents of irritation and respiratory disease are obscure. Thus, from a health effects viewpoint it remains uncertain whether the EHS investigator should sample during final clearance for total spores, culturable spores, hyphal fragments, specific allergens, glucans, endotoxins, or other agents.
Therefore, in an effort to ensure that one is using valid standard industry practices, from legitimate medical and scientific sources, legitimate mold assessors should continue to exclusively perform visual inspections as the preferred method of final clearance. Naturally, there will be some isolated (rare) cases, wherein an Industrial Hygienist will need to perform (expensive) air sampling to meet specific a priori DQOs.
BULK SAMPLES
Bulk samples usually provide virtually no useful information in assessments over and above that which can be identified by a cognizant expert by the naked eye. Bulk samples are seldom collected by cognizant experts. During our assessments, without the collection of any samples, we are usually able to provide the same information by merely looking at building materials. Even then, knowing the genus or species of a mold is usually of no value since the genus or the species of mold does not alter any subsequent decisions.
In general, we find that the bulk samples collected by "certified" mold inspectors and Home Inspectors are meaningless and useless, and are never actually used in any kind of decision making process. We have never seen a report by a mold tester that actually explained why knowing the genus was important to the process or how the genera involved impacted the remediation in anyway.
The same PARCC parameters (precision, accuracy, comparability, etc) described above for air samples also apply for bulk samples (tape lifts, bulks and swabs).
GIMMICKS
Home Test Kits and snake-oil gimmicks are often used by poorly trained and unethical inspectors to bamboozle their victims into thinking that something very scientific is being done, when in fact, the process is more of a dog-and-pony show without demonstrable benefits.
Examples would include the "InstaScope," HealthfulHome "The 5-Minute Mold" test, and the "IAQ Pro 5-Minute Home Mold Test." Like many of the mold inspection scams, the inspection often includes a device whose operation theories are sound and operates exactly as the manufacturer claims. For example, the analysis principles of the ERMI, the "Mycometer," and the "InstaScope" may all operate exactly as the manufacturer claims, however it's their utility in the application on an inspection that is usually without foundation.
Thus, for example, when researchers developed a new solid phase microextration GCMS /GCFID method for MVOCs, nobody disputed that the analytical technology worked as described what was disputed was the fact the method could not be demonstrated to have any utility during a mold inspection over and above a simple visual inspection.
As such, when we see individuals and laboratories known for their fraudulent behavior and deceptive trade practices as proponents of new "gee-whiz" technologies, usually there is some degree of snake-oil incorporated into the sales pitch.
The do-it-yourself mold tests on the market, such as the EPA "ERMI" kit and IAQ Pro 5-Minute Home Mold Test are misleading, and cannot be used to determine if an home has a problem.
The misleading assumption associated with products like the IAQ Pro 5-Minute Home Mold Test is that the product determines if a home has a mold problem, and the selling point is that the "analysis" is very accurate. However, having a sensitive or accurate analysis method is meaningless if the sample from which the analysis is derived is not capable of determining the significance of the result. Such is the case with the IAQ Pro 5-Minute Home Mold Test.
Since ALL houses without exception, contain "black molds" and we know that ALL houses, without exception contain Aspergillus and Penicillium and Stachybotrys, and the IAQ Pro 5-Minute Home Mold Test only tells us that these are present, what is the point of the "test?"
Similar DIY mold testing kits, such as the PRO-LAB Test Kits are merely settling plates marketed by Pro-LabsTM and sold through Home DepotTM and other retail outlets.
The IAQ Pro 5-Minute Home Mold Test, and similar DIY mold test products are NOT capable of determining the significance of the presence of the molds. These devices are entirely and completely incapable of producing any legitimate results under all circumstances and cannot be interpreted by anyone under any known conditions.
The public is generally mislead by the use of popular indices that claim to provide a moldiness index. Some of the more popular (and most misleading) are the US EPA "Environmental Relative Moldiness Index (ERMI)" and the EMLab P&K MoldScoreTM. These kinds of indices and scores have been grossly misapplied and have been used by poorly trained "mold inspectors" as some kind of a magical score to evaluate moldiness.
A study that appeared in the January 2015 edition of the Journal of Occupational and Environmental Hygiene 75 looked at the ERMI scores and attempted to determine if the ERMI correlated with health problems, such as "wheeze" in homes. What the authors found was that there was no statistically significant difference in ERMI scores in homes of children with wheeze, and children without wheeze.
In fact a primary finding of the 2015 study was that one could perform a visual inspection of the property and determine if the ERMI score would be high or low. That is, a visual inspection is just as good as wasting one's money on suspect protocols such as ERMI.
In fact, virtually all the claims surrounding the application of the ERMI score and the MoldScoreTM as being capable of identifying a problem are false (and always have been false). Neither index is accepted by science, and the problem of misapplying the US EPA ERMI score became such a problem, that in August 2013 the US EPA released a report 72a to address the misapplication and even the fraudulent misrepresentations being made about ERMI.
On January 2, 2014, FACTs did an internet search on companies offering the ERMI protocol, and every organization, without a single exception, that we found on the internet that was offering the protocol, falsely described the protocol and misrepresented the service and the ERMI protocol.
According to the US EPA:
The EPA readily acknowledged that MSQPCR [Mold Specific Quantitative Polymerase Chain Reaction] and ERMI have not been validated or peer reviewed by EPA for public use. The agency considers MSQPCR and ERMI to be research tools not intended for public use. However, the manner in which one active and one inactive licensee advertised MSQPCR and ERMI has the potential to mislead the public into thinking that these research tools are EPA-approved methods for evaluating indoor mold. Also, information appearing on an EPA Office of Science Policy webpage suggests that the EPA has validated and endorsed MSQPCR for public use. The EPA has developed but has not finalized a fact sheet on MSQPCR, ERMI and indoor mold for the public.Consequently, there is a risk that the public may make inappropriate decisions or take unnecessary actions regarding indoor mold on the belief that MSQPCR and ERMI results were based on research tools fully validated and endorsed by the EPA for public use.
In general, because the protocols sound "scientific" and because poorly trained field practitioners (almost exclusively "certified mold inspectors") were fraudulently claiming the method was an EPA standard or a EPA validated method, the public continues to be duped by charlatans offering the ERMI service.
Currently, NONE of the mold related indices or scores are capable of determining if a property has a mold problem.
Similarly, there is the "InstaScope," which was recently promoted in Colorado by an individual, Mr. Joe Boatman, with "Quality Environmental Services" (and associated with "Indoor and Outdoor Air Quality Consulting"). Mr. Boatman has an extended history of knowingly performing fraudulent property inspections, and making falsified claims in his reports; due to his gross incompetence, his deceptive inspections have resulted in thousands of regulatory violations in Colorado. 73, 74, 75, 76, 77, 78, 79
The "InstaScope" device may, as claimed count particles and even classify particles, but there is no documentation that the device has any utility in the performance of a legitimate mold inspection in a building whose ultimate goal is to correct a moisture problem and resolve visible mold growth in occupied spaces.
"Mold inspectors" have been losing law suit after law suit and have become embarrassed about the public's increasing awareness that their "testing" is not valid and have started to claim that their tests are for "screening purposes." However, this claim is also invalid and their "testing" methods are not appropriate for "screening." We have provided a discussion on the fallacy of "screening samples" here. CONCLUSION
Sampling and testing in mold assessments is virtually never needed, and virtually never provides any information that is not otherwise immediately available to a legitimate Industrial Hygienist.
Overall, most legitimate mold experts seldom see a need to collect any kind of samples. Usually, the only consultants who routinely collect mold samples or conduct mold tests are those consultants who need the laboratory report to achieve an image of credibility with the client, since they otherwise lack legitimate knowledge in the subject area.
Knowing the species and/or genus is virtually never useful information.
It is impossible, outside the context of a priori DQOs, to compare indoor samples and outdoor samples.
Virtually ALL of the hundreds of samples FACTs has reviewed over the years collected by Home Inspectors and "certified mold remediators" or "certified mold inspectors were useless or meaningless (or both), and always unnecessary.
Educational Videos
FACTs provides on-site training in several areas including radiation toxicology and indoor molds. Below is a six-part series on a two hour presentation on the myths of residential molds. The two hour talk is a simple, fact-based discussion of the myths of residential molds. The last two segments will be posted in April 2016.
An Introduction to Molds
Molds! Part 2 Health Effects
Myths of Sampling 3A
Myths of Sampling 3B
Recognizing a Problem
Correcting the Problem
About the Author
Mr. Connell has been a continuously practicing Industrial Hygienist since 1987. Prior to entering the Industrial Hygiene field, he had approximately ten years experience as an analytical chemist in analytical and research laboratories in the United States and in Ireland.
Mr. Connell regularly performs legitimate mold "testing" (mold assessments) around the U.S. and has performed approximately 900 mold and microbial assessments during the last 25 years. Mr. Connell has performed microbial investigations in a number of litigious cases and for such highly acclaimed organizations as the National Center for Atmospheric Research (where Mr. Connell has served as the contract Industrial Hygienist for approximately ten years). He has been qualified as an expert witness in Federal Court (Philadelphia)81 on the lack of scientific validity of common mold sampling and testing used by "certified" mold inspectors. He has similarly been used as an expert witness in mold issues in Federal Criminal Court82 (US Bureau of Alcohol Tobacco and Firearms, in Federal Court, District of Colorado) and has provided testimony in several indoor air and mold related cases.
Mr. Connell's clients have included the U.S. Geological Survey, Health and Human Services, Federal Bureau of Prisons, and the National Institute of Standards and Technology.
Mr. Connell is a member of the American Industrial Hygiene Association (AIHA), the American Conference of Governmental Industrial Hygienists (ACGIH), the Property Care Association (England) and the Occupational Hygiene Society of Ireland, and he currently serves on three International Standards Committees including ASTM D22.08 Indoor Air (whose task is to develop and write internally accepted indoor and building mold assessment standards); ASTM E50 Committee (Environmental Assessment & Risk Management), and ASTM E30.05 Forensic Sciences committee. Mr. Connell serves as an Industrial Hygiene Subject Matter Expert for the Federally funded Inter Agency Board (Health, Medical Responder Safety Subgroup).
Mr. Connell is an internationally recognized expert on indoor microbial issues and has lectured on risk assessment, sampling and toxicology at the university level. In 2016, he was a guest lecturer at the Moller Centre (Churchill College, in the University of Cambridge, England) and in 2011, he was a guest lecturer for the PCA (Huntingdon, England), he has also lectured at University of Vermont, (Johnson Conference) University of Arizona (Arizona Health Sciences Center, Zuckerman College of Public Health), University of Colorado, and the Environmental Information Association. He has presented papers on the statistical variations associated with indoor mold sampling including the international symposium on indoor mold at the International Johnson Conference at the University of Vermont where he served as Committee Chairman on the Mold Health Effects Committee.
References
1a Robertson LD, et al A multi-laboratory comparative study of spore trap analyses Mycologia,103(1), 2011, pp. 226-231. DOI: 10.3852/10-017
1 Macher JM, Chatigny MA, Burge HASampling airborne microorganisms and aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617.
2 American Industrial Hygiene Association
3 Recognition, Evaluation, and Control of Indoor Mold, Prezant E; Weekes, DM; Miller JD (Eds.) American Industrial Hygiene Association 2008
4 The CDC Mold Work Group, National Center for Environmental Health, National Center for Infectious Diseases, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, October 2005
5 US Centers for Disease Control, Mold: General Information: Basic Facts | CDC APRHB, 2007, http://www.cdc.gov/mold/faqs.htm
6 Watson JG, Turpin BJ Chow JC, The Measurement Process; Precision, Accuracy and Validity, Chapter 10 in Air Sampling Instruments for Evaluation of Atmospheric Contaminants (ACGIH, 2001)
7 US Centers for Disease Control, Mold: General Information: Basic Facts | CDC APRHB, 2007, http://www.cdc.gov/mold/faqs.htm
8 US EPA Test Methods for Evaluating Solid Waste, Physical/Chemical Methods, 1996 is OSW's official compendium of analytical and sampling methods that have been evaluated and approved for use in complying with the RCRA regulations.
9 Li Y; Heng J; and Chen Z Study Of Particle Movement In Ventilation System Proceedings: Indoor Air 2002 Anaheim California, 2002
10 Keady PB; Mainquist L; Tracking IAQ Problems to Their Source, Occupational Health & Safety, September 2000
11 Connell CP, Field Measurements for Moulds: Spatial and Temporal Variations, Presented at the ASTM International Conference: Bringing Science to Bear on Moisture and Mold in the Built Environment, Colorado University, Boulder 2006
12 Macher JM, Chatigny MA, Burge HA Sampling airborne microorganisms and aeroallergens In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617.
13 Ayer HE; Burg J, Time Weighted Averages Vs. Maximum Personal Sample (Presented at the AIHA Conference, Boston, MA, 1973)
14 NIOSH Occupational Exposure Sampling Strategy Manual, HEW Publication Number 77-173 (1977)
15 Spurgeon, J; Data submitted to the ASTM D22.08.02 Committee for review, October 2005
16 Connell, CP, Sample results: What do they really tell us? Presented at the IAQ in Schools Lecture Series, Corpus Christi, TX, 2003
17 Eudey L, Su HJ, Burge HA. Biostatistics and bioaerosols. In Bioaerosols, Burge HA, ed. Boca Raton: Lewis Publishers, pp. 269-307. 1995.
18 Reponen T, Nevalainen A, Jantunen M, et al, Normal Range Criteria for Indoor Air Bacteria and Fungal Spores in a Subarctic Climate; Indoor Air, 2:26-31 (1992). Referenced by Macher JM, Chatigny MA, Burge HA. Sampling airborne microorganisms and aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617, but not reviewed by this author (Connell).
19 NIOSH Occupational Exposure Sampling Strategy Manual, HEW Publication Number 77-173 (1977)
20 Ibid.
21 As referenced in Rock JC; Occupational Air Sampling Strategies, Chapter 2 of Air Sampling Instruments for Evaluation of Atmospheric Contaminants (ACGIH, 2001)
22 NIOSH Technical Information Exposure Measurement Action Level and Occupational Environmental Variability, HEW Publication 76-131, Cincinnati OH, 45226, (1975)
23 NIOSH Occupational Exposure Sampling Strategy Manual, HEW Publication Number 77-173 (1977)
24 Macher JM, Chatigny MA, Burge HA Sampling airborne microorganisms and aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617.
25 Ibid.
26 Shapiro-Wilk W test (used to determine the most appropriate data distribution curve) greater than 0.9500.
27 In the above closed-mode building data set, the one-tail percentage point of the W test =0.7880; the Shapiro-Wilk W test (goodness of fit) is 0.9890, indicating lognormal distribution (Gaussian distribution is rejected since W for normal is 0.7800). There is 95% confidence any single randomly collected sample will exceed 1,000 spores/m3 65% of the time; the MVUE is 1,058 spores/m3 with a GSD of 2.6
28 Generally speaking, and certainly at FACTs, because of the expected variations, we express the "average" value as the MVUE.
29 Based on the air volumes reported in typical laboratory reports, collected single 3-minute samples.
30 Heber AJ, Bioaerosol Particle Statistics in Chapter 5, of Cox CS, Wathes CM Bioaerosols Handbook 1995
31 Macher JM, Chatigny MA, Burge HA Sampling airborne microorganisms and aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of atmospheric contaminants, 8th ed. Cincinnati, OH: American Conference of Governmental Industrial Hygienists, Inc., pp. 589-617. 1995
32 Ibid.
33 Macher J. Burge HA, Sampling Biological Aerosols Chap. 22 in Air Sampling Instruments for Evaluation of Atmospheric Contaminants (ACGIH, 2001)
33a Robertson LD, et al A multi-laboratory comparative study of spore trap analyses Mycologia, 103(1), 2011, pp. 226-231. DOI: 10.3852/10-017
34 Alegro Industries, Operator's Manual, IS013 Rev E 7-7-05 (Allegro Industries, 7221 Orangewood Avenue, Garden Grove, CA 92841)
35 Saulius T, Willeke K, Reponen T, Trunov M, Particle Cut-Size Evaluation Final Report Nov 1998, Internal Report by Zefon International-Analytical Accessories, 2860 23rd Ave, St. Petersburg, FL, 33713
36 Cadle RD The Measurement of Airborne Particles (1975), (referencing seminal work by Ludwig, FL Env. Sci. Technology 2, 1968).
37 Connell CP, Sample Results: What do they really tell us? IAQ Sampling Myths 13th Annual AIHA/ASSE OEH&S Conference, "Exchanging Knowledge, New Times, New Ideas" Denver, CO October 2007
37a Robertson LD, et al A multi-laboratory comparative study of spore trap analyses Mycologia, 103(1), 2011, pp. 226-231. DOI: 10.3852/10-017
38 Burge HA, Bioaerosol Investigations, Chapter 1 in Bioaerosols Burge HA (ed), 1995
39 Burge HA Bioaerosols in the Residential Environment , Chapter 21 in Bioaerosols Handbook (Cox CS, Wathes CM eds), 1995
40 NIOSH is the US Department of Health and Human Services, Centers for Disease Control, National Institutes of Occupational Safety and Health.
41 Muilenburge ML, The Outdoor Aerosol, in Chapter 9 of Bioaerosols, (Burge HA, ed) 1995
42 Madelin TM, Madelin MF Biological Analysis of Fungi and Associated Molds; Bioaerosols Handbook, Cox and Wathes, Eds. (1995)
43 Chapter J - Sampling and Characterization of Bioaerosols; NIOSH Manual of Analytical Methods (NMAM), 4th ed. DHHS (NIOSH) Publication 94-113 (August, 1994), 1st Supplement Publication 96-135, 2nd Supplement Publication 98-119, 3rd Supplement 2003-154
44 NIOSH Method 0800, BIOAEROSOL SAMPLING (Indoor Air) Culturable organisms: bacteria, fungi, thermophilic actinomycetes, Issue 1, January 1998
45 Mold Remediation in Schools and Commercial Buildings U.S. Environmental Protection Agency (EPA 402-K-01-001, March 2001 updated June 25, 2001)
46 December data from an "healthy" Massachusetts residence with some visible mold
47 Connell CP, Field Measurements for Moulds: Spatial and Temporal Variations, Presented at the ASTM International Conference: Bringing Science to Bear on Moisture and Mold in the Built Environment, Colorado University, Boulder 2006
48 The statistic known as a "minimum variance unbiased estimate" (MVUE) is considered to be a statistic that best represents a point estimate of the true "mean" for lognormally distributed values. The MVUE is preferred over the geometric mean, especially when sample populations are small.
49 Muilenburge ML, The Outdoor Aerosol, in Chapter 9 of Bioaerosols, (Burge HA, ed) 1995
50 Least squares fit, r2 = 0.058
51 Cooley J.D.; Wong W.C.; Straus D.C.; Jumper C.A. Correlation between the prevalence of certain fungi and sick building syndrome, Occupational and Environmental Medicine, September 1998, vol. 55, no. 9, pp. 579-584(6)
52 El-Hougeiri N., El-Fadel M.IAQ Characterization In Urban Areas: Indoor To Outdoor Correlation Proceedings: Indoor Air 2002
53 Connell CP, Field Measurements for Moulds: Spatial and Temporal Variations, Presented at the ASTM International Conference: Bringing Science to Bear on Moisture and Mold in the Built Environment, Colorado University, Boulder 2006
54 Shelton BG, Kirkland KH, Flanders WD, Morris GK, Profiles of American Fungi in Buildings and Outdoor Environments in the United States Applied and Env. Microbiology April 2000 pp 1743-1753
55 Connell CP, Indoor Fungal Concentrations http://www.forensic-applications.com/moulds/mvue.html
56 Closed mode conditions.
57 n=136. Data exceeding the upper quantifiable limit (estimated to be 1.7E6 spores/m3) were censored to 1.7E6 spores/m3.
58 American Academy of Allergy and Immunology http://www.aaaai.org/nab/index.cfm?p=reading_charts
59 Gravensen, S. Fungi as a cause of allergic disease Allergy (34): 135-154, 1979; as reported in Ren P; Jankun TM; Leaderer BP; Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county Journal of Exposure Analysis and Environmental Epidemiology (9) 560-568; 1999
60 Robbins C, Morrell J; Mold, Housing and Wood(Article prepared for the Western Wood Products Association), Jan 2006.
61 Daggett DA, Chamberlain M, Smith W. Effects of Exterior Decay and Mold on Indoor Mold and Air Quality. Proceedings of the 2nd Annual Conference on Durability and Disaster Mitigation: November 6, 2000; Madison, WI (Cited by Robbins as referenced here, but not reviewed by this author (Connell))
62 Burge, H. Can Mold Be Safely Left Inside Walls? The Environmental Reporter, Vol. 3, No. 11, November 2005
62a Preventing Occupational Respiratory Disease from Exposures caused by Dampness in Office Buildings, Schools, and Other Nonindustrial Buildings, DHHS (NIOSH) Publication No. 2013-102, November, 2012
63 Recognition, Evaluation, and Control of Indoor Mold, Prezant E; Weekes, DM; Miller JD (Eds.) American Industrial Hygiene Association 2008, Section 2, Chapter 9
64 Health Canada: Fungal Contamination in Public Buildings: Health Effects and Investigation Methods. Health Canada, Ottawa, ON (2004)
65 Canadian Construction Association; Mould Guidelines for the Canadian Construction Industry; CCA; Ottawa, ON; 2004
66 Guidelines on Assessment and Remediation of Fungi in Indoor Environment; New York City Department of Health, Bureau of Environmental & Occupational Disease Epidemiology, 2000
67 Guidelines on Assessment and Remediation of Fungi in Indoor Environment; New York City Department of Health, Bureau of Environmental & Occupational Disease Epidemiology, 2000
68 Health Canada: Fungal Contamination in Public Buildings: Health Effects and Investigation Methods. Health Canada, Ottawa, ON (2004)
69 Canadian Construction Association; Mould Guidelines for the Canadian Construction Industry; CCA; Ottawa, ON; 2004
70 US Environmental Protection Agency, in its booklet "Mold Remediation in Schools and Commercial Buildings, EPA 402-K-01-001 March 2001 (updated 6/25/01)
71 American Conference of Governmental Industrial Hygienists, (ACGIH), Data Interpretation, In Bioaerosols: Assessment and Control, Macher J (Ed), Cincinnati OH, 1999
72 Storey E; et al Guidance for Clinicians on the Recognition and Management of Health Effects Related to Mold Exposure and Moisture Indoors, Farmington CT, University of Conn. Health Center, 2004
72a US Environmental Protection Agency, Office Of Inspector General Report No. 13-P-0356, August 22, 2013 "Public May Be Making Indoor Mold Cleanup Decisions Based on EPA Tool Developed Only for Research Applications" See also: MEMORANDUM: THE INSPECTOR GENERAL, from Arthur A. Elkins Jr. US EPA (August 22, 2013)
73 769 Cleveland Cir, Lafayette, CO, (189 Regulatory Violations) http://www.forensic-applications.com/meth/Boatman_Cleveland_PA_RA.pdf
74 731 Excelsior Place, Lafayette, CO 80026 (344 Regulatory violations) http://forensic-applications.com/meth/Boatman_Excelsior_PA_RA.pdf
75 502C West South Boulder Road, Louisville, CO 80027 (PA) (357 Regulatory violations) 502C West South Boulder Road, Louisville, CO 80027 (PA) http://forensic-applications.com/meth/Boatman_502C_PA_RA.pdf
76 1815 Regal Ct., Unit B, Louisville, CO 80027 (90 Regulatory violations) http://www.forensic-applications.com/meth/Boatman_Screening_Regal_RA.pdf
77 1815 Regal Ct., Unit B, Louisville, CO 80027 (234 Regulatory violations) http://www.forensic-applications.com/meth/Boatman_Regal_PA_RA.pdf
78 1815 Regal Ct., Unit B, Louisville, CO 80027 (357 Regulatory Violations) http://forensic-applications.com/meth/Boatman_Regal_Clearance_RA.pdf
79 2330 Wedgewood Ave., Building 7, Longmont, CO 80503 (345 Regulatory violations) http://forensic-applications.com/meth/Boatman_Screening_Wedge7_RA.pdf
80 Institute of Medicine (IOM) Damp Indoor Spaces and Health, DC, IOM, 2004
81 220 W. Rittenhouse Square Condominium Association v. Myrna Stolker. Philadelphia CCP April Term 2009 No. 02446, Honorable Gary F. Di Vito presiding (May 2012)
82 US v. Stylio Trachanas Case No. 2011VR00678, DJJ-12W-USA13-0136, July 9, 2012
83 Rosenbaum PF, Crawford JA, Hunt A, Vesper SJ, Environmental Relative Moldiness Index and Associations with Home Characteristics and Infant Wheeze. Journal of Occupational and Environmental Hygiene, 12: 29-36 ISSN: 1545-9624 print / 1545-9632 online Copyright 2015 JOEH, LLC DOI: 10.1080/15459624.2014.933958
This page was originally created on April 25, 2009 and was last revised on April 2022.
Visitors to this page generally have an interest in scientific issues. If you are interested in such matters, you may find some of our other discussions interesting.
To visit our page concerning air monitoring aspects of moulds, click here.
To visit our page concerning health aspects of moulds (myth busting), click here.
A discussion concerning myths surrounding duct cleaning, can be found by clicking here.
A discussion concerning meth-labs and illegal drug labs, can be found by clicking here.
For issues surrounding the history and cause of carpal tunnel syndrome click here.
For a discussion concerning indoor radon click here.
For a discussion concerning indoor air quality, click here.
For a discussion concerning the myths associated with laboratory fume hood face velocities click here.
For a discussion concerning laboratory fume hood evaluations, click here.
![[logojpg]]( http://www.forensic-applications.com/greybutton.jpg) | Forensic Applications Consulting Technologies, Inc. |
Feel free to send Forensic Applications, Inc. an email directly by clicking here
![[185 Bounty Hunter's Lane, Bailey, Colorado]](http://www.forensic-applications.com/addressjpg.jpg)